Answer:
(i) ?![]() group
is electron-withdrawing group and thus, stability of nitrophenoxide is further
increased.
-
group
is electron-withdrawing group and thus, stability of nitrophenoxide is further
increased.
-![]() group
is electron-repelling group which decreases stability of methoxy peroxide ion.
group
is electron-repelling group which decreases stability of methoxy peroxide ion.
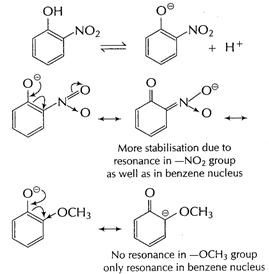 Thus, o-nitro phenol is more acidic than o-methoxyphenol.
[1]
Thus, o-nitro phenol is more acidic than o-methoxyphenol.
[1]
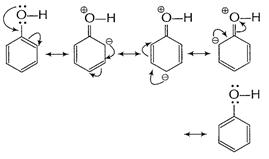 Due to resonance electron-density at o ? and p-positions
is increased.
Resonance hybrid of the above structures is given below
Due to resonance electron-density at o ? and p-positions
is increased.
Resonance hybrid of the above structures is given below
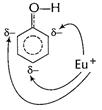 Electrophile
Electrophile ![]() can
attack at these sites which are o- and p- wrt to ?OH group.
Thus, ?OH group activates benzene nucleus for o- and
p-attack. [1]
can
attack at these sites which are o- and p- wrt to ?OH group.
Thus, ?OH group activates benzene nucleus for o- and
p-attack. [1]
You need to login to perform this action.
You will be redirected in
3 sec
