Answer:
(i) Mole fraction Mole fraction of a component (1) is
the ratio of the moles of that component (1) and that of total moles in the
mixture of (1) and (2) or more
![]() moles
of component (1)
moles
of component (1)
![]() moles
of component (2)
moles
of component (2)
![]() total
moles
Then mole fraction of the components (1) and (2) are
total
moles
Then mole fraction of the components (1) and (2) are
![]()
![]()
![]() In general
In general
![]() (component
i)
(component
i)![]() [1]
(ii) Molality is the concentration in moles of solute per
kg of solvent.
[1]
(ii) Molality is the concentration in moles of solute per
kg of solvent. ![]() g
solute is present in
g
solute is present in![]() solvent.
Moles of solute
solvent.
Moles of solute![]() Solvent
Solvent ![]() Then molality
Then molality ![]()
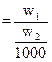 molality
molality![]() Mole fraction and molality both are not affected by change in
temperature. [1]
Mole fraction and molality both are not affected by change in
temperature. [1]
You need to login to perform this action.
You will be redirected in
3 sec
