Answer:
![]() (depression
in freezing point)
(depression
in freezing point)
![]()
![]()
![]() moles
of solute
moles
of solute![]()
![]() grams
of solvent
grams
of solvent ![]() Molality = mol
Molality = mol ![]()
![]() [1]
i = van't Hoff factor
[1]
i = van't Hoff factor
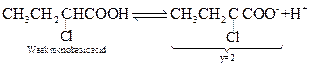
![]() By Ostwald's dilution law
By Ostwald's dilution law
![]()
![]()
![]()
![]() [1]
[1]
![]()
![]()
![]() [1]
[1]
You need to login to perform this action.
You will be redirected in
3 sec
