Answer:
(a) (i)
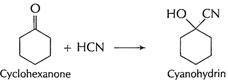
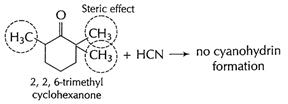 In cyclohexanone, cyanohydrin is formed easily as there is
no steric hindrance. But in 2, 2, 6- tri methyl cyclohexanone, adjacent
positions are occupied. This produces steric hindrance and thus approach of
HCN, in nucleophilic addition is prevented. [1]
(ii)
In cyclohexanone, cyanohydrin is formed easily as there is
no steric hindrance. But in 2, 2, 6- tri methyl cyclohexanone, adjacent
positions are occupied. This produces steric hindrance and thus approach of
HCN, in nucleophilic addition is prevented. [1]
(ii)
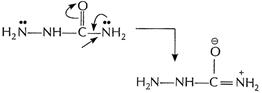 Lone pair on this N is delocalized due to presence of
CO group. Thus, it is less basic as compared to
Lone pair on this N is delocalized due to presence of
CO group. Thus, it is less basic as compared to ![]() attached
to NH.
attached
to NH.
 [1]
(iii)
[1]
(iii) 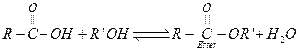
![]() (alkoxide)
is a better leaving group. If
(alkoxide)
is a better leaving group. If![]() or ester
is not removed, above reaction becomes reversible and yield of the ester
(product) is decreased. [1]
(b)
or ester
is not removed, above reaction becomes reversible and yield of the ester
(product) is decreased. [1]
(b)
![]()
![]() When
When ![]() and
and ![]() both take
part in one reaction.
both take
part in one reaction.
![]() acts
as nucleophile then product is
acts
as nucleophile then product is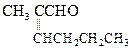
![]()
![]() acts
as nucleophile then product is
acts
as nucleophile then product is
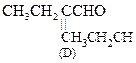
![]() Or
(a) (i) Benzaldehyde to 3-phenyl propan -1-ol
Or
(a) (i) Benzaldehyde to 3-phenyl propan -1-ol
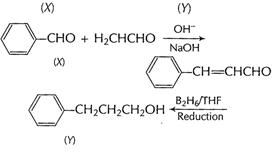 [1]
(ii) Benzoic acid (X) to m-nitro benzyl alcohol (Y)
[1]
(ii) Benzoic acid (X) to m-nitro benzyl alcohol (Y)
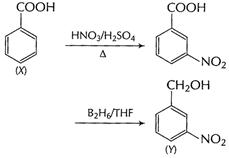 [1]
(iii) Propanone (X) to propene (Y)
[1]
(iii) Propanone (X) to propene (Y)
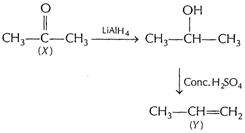 [1]
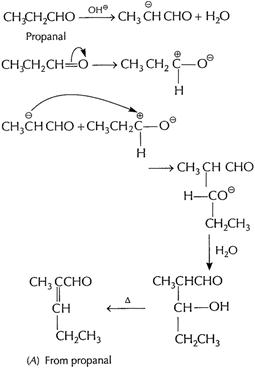
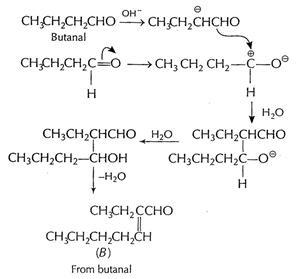
(b) (i) Cross aldol condensation When two different
aldehydes having at least one a - H are treated with NaOH, there is formation
of aldol.
One of the aldehyde acts as nucleophile and condensation
product can be converted into enal. This type of reaction involving different aldehydes
is called cross aldol condensation.
Ketones can also undergo this reaction.
[1]
(b) (i) Cross aldol condensation When two different
aldehydes having at least one a - H are treated with NaOH, there is formation
of aldol.
One of the aldehyde acts as nucleophile and condensation
product can be converted into enal. This type of reaction involving different aldehydes
is called cross aldol condensation.
Ketones can also undergo this reaction.
![]() From
From![]() +
From
+
From ![]() +
From A and B
+
From A and B![]() +
+
![]() [1]
(ii) Decarboxylation Sodium salt of carboxylic acids
on heating with sodalime changes to hydrocarbon with one carbon less.
This is called decarboxylation of the acid.
[1]
(ii) Decarboxylation Sodium salt of carboxylic acids
on heating with sodalime changes to hydrocarbon with one carbon less.
This is called decarboxylation of the acid.
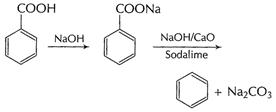 Decarboxylation is used for descending a chain.
Decarboxylation is used for descending a chain.
 [1]
[1]
You need to login to perform this action.
You will be redirected in
3 sec
