Answer:
![]() is
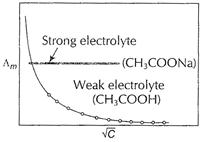
a strong electrolyte. At high concentration, there are greater number of ions
per unit volume which increases attraction between
is
a strong electrolyte. At high concentration, there are greater number of ions
per unit volume which increases attraction between ![]() and
and ![]() ions. If
solution is diluted, then it decrease in ionic attractions, hence molar conductivity
increases with dilution, on regular basis.
On the other hand,
ions. If
solution is diluted, then it decrease in ionic attractions, hence molar conductivity
increases with dilution, on regular basis.
On the other hand, ![]() is a
weak acid. By Ostwald's dilution law, degree of ionization (x) is related to
dilution by
is a
weak acid. By Ostwald's dilution law, degree of ionization (x) is related to
dilution by ![]() [1]
In higher concentration range, ionisation is very small,
thus molar conductance, also remains constant. In lower concentration range
(very dilute solution) ionisation is rapid, hence molar conductance
increases rapidly with dilution.
[1]
In higher concentration range, ionisation is very small,
thus molar conductance, also remains constant. In lower concentration range
(very dilute solution) ionisation is rapid, hence molar conductance
increases rapidly with dilution.
 [1]
[1]
![]() (infinite
dilution)
(infinite
dilution)
You need to login to perform this action.
You will be redirected in
3 sec
