| Experiment |
time |
Total pressure (atm) |
| I II | 0 100 | 0.5 0.6 |
 (i) What is the order of the reaction?
(ii) What are the units of rate constant, k for the
reaction? [1+3+1]
(i) What is the order of the reaction?
(ii) What are the units of rate constant, k for the
reaction? [1+3+1]
Answer:
![]() +
+![]() Initial at a 0 0
T = 0 time (a - x) x x
[1]
Initial pressure is due to
Initial at a 0 0
T = 0 time (a - x) x x
[1]
Initial pressure is due to ![]() atm
atm ![]() At time t (100 s) pressure is due to
At time t (100 s) pressure is due to ![]() and
and![]() Thus,
Thus, ![]()
![]() x = 0.6 - 0.5
= 0.1 atm [1]
(a - x)
x = 0.6 - 0.5
= 0.1 atm [1]
(a - x) ![]() 0.5 - 0.
1 = 0.4 atm
0.5 - 0.
1 = 0.4 atm ![]() Thus,
Thus, ![]()
![]()
![]() [2]
Or
(a)
[2]
Or
(a)![]()
![]()
![]()
![]()
![]()
![]() [1]
(b)
[1]
(b)![]() (half-life
period) = 3 hrs
(half-life
period) = 3 hrs
![]() After 8 hrs
After 8 hrs
![]()
![]()
![]()
![]()
![]() [3]
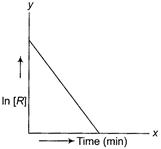
(c) Coordinates involve In [R] terms.
Thus it may be first order reaction.
[3]
(c) Coordinates involve In [R] terms.
Thus it may be first order reaction.
![]()
![]()
![]()
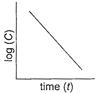 (i) Order =1
(i) Order =1 ![]() (ii) Unit of rate constant=time-1
(ii) Unit of rate constant=time-1 ![]()
You need to login to perform this action.
You will be redirected in
3 sec
