Answer:
(a)
Phenol has smaller dipole moment than methanol due to electron withdrawing
effect of phenyl group. As a result the ![]() in phenol
is less polar.
in phenol
is less polar. ![]() On
the other hand, in methanol the methyl group is electron releasing effect and
hence
On
the other hand, in methanol the methyl group is electron releasing effect and
hence ![]() group is
more polar and dipole moment of phenol is higher than methanol.
group is
more polar and dipole moment of phenol is higher than methanol. ![]() (b)
Phenyl group is electron withdrawing in nature. Therefore electron density
on oxygen of
(b)
Phenyl group is electron withdrawing in nature. Therefore electron density
on oxygen of ![]() group
decreases. So, it is easily protonated.
In
case of alcohols, the alkyl group is
group
decreases. So, it is easily protonated.
In
case of alcohols, the alkyl group is ![]() effect
group which increases the
effect
group which increases the ![]() density on
oxygen atom. Therefore it is not easily protonated.
1
(c)
Alcohols acts as weak bases due to presence of lone pair on oxygen atom.
density on
oxygen atom. Therefore it is not easily protonated.
1
(c)
Alcohols acts as weak bases due to presence of lone pair on oxygen atom.
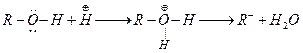 1
1
You need to login to perform this action.
You will be redirected in
3 sec
