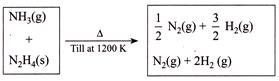
 \[{{P}_{i}}=0.3\,atm\] \[{{P}_{f}}=2.7\,atm\] \[{{T}_{i}}=300\,K,\] \[{{T}_{f}}=1200\,K\] \[{{V}_{i}}=VL\] \[{{V}_{f}}=VL\] The \[mol\,%\]of \[N{{H}_{3}}\]in the original mixture is
\[{{P}_{i}}=0.3\,atm\] \[{{P}_{f}}=2.7\,atm\] \[{{T}_{i}}=300\,K,\] \[{{T}_{f}}=1200\,K\] \[{{V}_{i}}=VL\] \[{{V}_{f}}=VL\] The \[mol\,%\]of \[N{{H}_{3}}\]in the original mixture is
A) \[25%\]
B) \[20%\]
C) \[75%\]
D) \[37.5%\]
Correct Answer: C
Solution :
[c] Using equation \[PV=nRT\]| Initial moles | Total final moles |
| \[{{n}_{1}}=\] moles of \[N{{H}_{3}}\] | Moles formed from \[N{{H}_{3}}=\left( \frac{1}{2}+\frac{3}{2} \right){{n}_{1}}\] |
| \[{{n}_{2}}=\] moles of \[{{N}_{2}}{{H}_{4}}\] | Moles formed from \[{{N}_{2}}{{H}_{4}}=(1+2){{n}_{2}}=3{{n}_{2}}\] |
You need to login to perform this action.
You will be redirected in
3 sec
