A) Halogenation of \[C{{H}_{4}}\]
B) Nitration of benzene
C) An alkene prepared by \[{{E}_{1}}CB\] mechanism
D) An alkene prepared by \[{{E}_{1}}\] mechanism
Correct Answer: A
Solution :
[a] (1) It shows \[{{1}^{{}^\circ }}\] isotope effect since \[(C-H)\] and \[(C-D)\] bonds broken in the halogenation of \[C{{H}_{4}}\] and \[C{{D}_{4}}\] \[{{H}_{3}}C-H\xrightarrow[hv]{C{{l}_{2}}^{+}}{{H}_{3}}C-Cl+HCl\] \[{{D}_{3}}C-D\xrightarrow[hv]{C{{l}_{2}}^{+}}{{D}_{3}}C-Cl+DCl\] (2) It does not show \[1{}^\circ \] isotope effect since no \[(C-H)\] or \[(C-D)\] bond breaks in first R.D.S. (3) It does not show \[1{}^\circ \] isotope effect. In \[E1cB,\] the reaction rate does not depend on the breaking of \[(C-H)\] or \[(C-D)\] bond but depends on the leaving \[{{X}^{\Theta }}\] ion in the second step.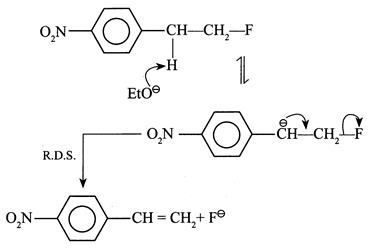 EWG \[[-N{{O}_{2}})\] group] in the compound and the poor leaving ability of \[{{F}^{\Theta }}\] makes the carbanion stable.
EWG \[[-N{{O}_{2}})\] group] in the compound and the poor leaving ability of \[{{F}^{\Theta }}\] makes the carbanion stable. 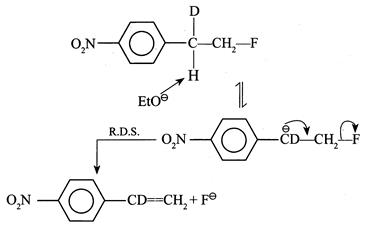 (4) In \[E1,\] the reaction takes place by the formation of \[3{}^\circ \]\[{{C}^{\oplus }}\] in the R.D.S. \[{{(C{{H}_{3}})}_{3}}C-X\xrightarrow{R.D.S.}{{(C{{H}_{3}})}_{3}}{{C}^{\oplus }}+{{X}^{\Theta }}\] \[{{(CD)}_{3}}C-X\xrightarrow{R.D.S.}{{(C{{D}_{3}})}_{3}}{{C}^{\oplus }}+{{X}^{\Theta }}\] Here, \[(C-H)\] or \[(C-D)\] bond is not broken, but the rate of reaction depends on the nature of the surrounding groups at \[(C-X)\] or the rate of reaction is slowed down if D is incorporated; hence it shows \[2{}^\circ \] isotope effect.
(4) In \[E1,\] the reaction takes place by the formation of \[3{}^\circ \]\[{{C}^{\oplus }}\] in the R.D.S. \[{{(C{{H}_{3}})}_{3}}C-X\xrightarrow{R.D.S.}{{(C{{H}_{3}})}_{3}}{{C}^{\oplus }}+{{X}^{\Theta }}\] \[{{(CD)}_{3}}C-X\xrightarrow{R.D.S.}{{(C{{D}_{3}})}_{3}}{{C}^{\oplus }}+{{X}^{\Theta }}\] Here, \[(C-H)\] or \[(C-D)\] bond is not broken, but the rate of reaction depends on the nature of the surrounding groups at \[(C-X)\] or the rate of reaction is slowed down if D is incorporated; hence it shows \[2{}^\circ \] isotope effect.
You need to login to perform this action.
You will be redirected in
3 sec
