A)
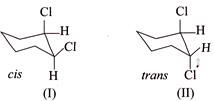 \[{{E}_{2}}\] elimination of \[HCl\] with \[M{{e}_{3}}COK+M{{e}_{2}}C-OH\] give
\[{{E}_{2}}\] elimination of \[HCl\] with \[M{{e}_{3}}COK+M{{e}_{2}}C-OH\] give 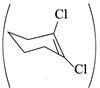 of (I) is faster than (II).
of (I) is faster than (II).
B)
C)
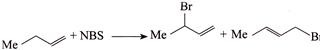
D)
Correct Answer: D
Solution :
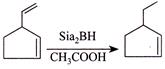
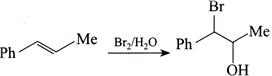
[d] (1) CORRECT It is faster since \[Cl\] H and \[Cl\] (two eliminating groups) are in anti-position. (2) CORRECT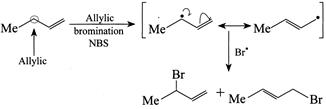 (3) CORRECT: - \[Si{{a}_{2}}BH\] add at less hindered double bond (4) INCORRECT:
(3) CORRECT: - \[Si{{a}_{2}}BH\] add at less hindered double bond (4) INCORRECT: 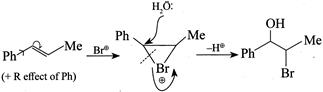 Product is different in the question.
Product is different in the question.
You need to login to perform this action.
You will be redirected in
3 sec
