A) \[{{\upsilon }_{3}}={{\upsilon }_{1}}-{{\upsilon }_{2}}\]
B) \[{{\lambda }_{3}}={{\lambda }_{1}}+{{\lambda }_{2}}\]
C) \[{{\lambda }_{3}}=\frac{{{\lambda }_{1}}{{\lambda }_{2}}}{{{\lambda }_{1}}+{{\lambda }_{2}}}\]
D) \[{{\upsilon }_{3}}=\frac{{{\upsilon }_{1}}{{\upsilon }_{2}}}{{{\upsilon }_{1}}+{{\upsilon }_{2}}}\]
Correct Answer: D
Solution :
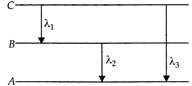
[d]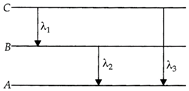 As is clear from figure, \[{{E}_{3}}={{E}_{1}}+{{E}_{2}}\] \[h{{\upsilon }_{3}}=h{{\upsilon }_{1}}+h{{\upsilon }_{2}}\]or\[{{\upsilon }_{3}}={{\upsilon }_{1}}+{{\upsilon }_{2}}\] From \[{{E}_{3}}={{E}_{1}}+{{E}_{2}}\] \[\frac{hc}{{{\lambda }_{3}}}=\frac{hc}{{{\lambda }_{1}}}+\frac{hc}{{{\lambda }_{2}}}\]or\[\frac{1}{{{\lambda }_{3}}}=\frac{1}{{{\lambda }_{1}}}+\frac{1}{{{\lambda }_{2}}}=\frac{{{\lambda }_{2}}+{{\lambda }_{1}}}{{{\lambda }_{1}}{{\lambda }_{2}}}\] \[{{\lambda }_{3}}=\frac{{{\lambda }_{1}}{{\lambda }_{2}}}{{{\lambda }_{1}}+{{\lambda }_{2}}}\]
As is clear from figure, \[{{E}_{3}}={{E}_{1}}+{{E}_{2}}\] \[h{{\upsilon }_{3}}=h{{\upsilon }_{1}}+h{{\upsilon }_{2}}\]or\[{{\upsilon }_{3}}={{\upsilon }_{1}}+{{\upsilon }_{2}}\] From \[{{E}_{3}}={{E}_{1}}+{{E}_{2}}\] \[\frac{hc}{{{\lambda }_{3}}}=\frac{hc}{{{\lambda }_{1}}}+\frac{hc}{{{\lambda }_{2}}}\]or\[\frac{1}{{{\lambda }_{3}}}=\frac{1}{{{\lambda }_{1}}}+\frac{1}{{{\lambda }_{2}}}=\frac{{{\lambda }_{2}}+{{\lambda }_{1}}}{{{\lambda }_{1}}{{\lambda }_{2}}}\] \[{{\lambda }_{3}}=\frac{{{\lambda }_{1}}{{\lambda }_{2}}}{{{\lambda }_{1}}+{{\lambda }_{2}}}\]
You need to login to perform this action.
You will be redirected in
3 sec
