A) 2 and 3
B) 3 and 3
C) 3 and 4
D) 4 and 3
Correct Answer: B
Solution :
[b] For X:- \[{{[Mabcd]}^{n\pm }}:\]: For complexes with general formula Mabcd, three geometrical isomers [a], [b] and [c] are possible.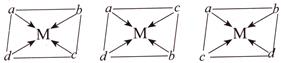
| A | B | C | |
| \[a-b\] | cis | trans | cis |
| \[c-d\] | cis | trans | cis |
| \[a-d\] | cis | cis | trans |
| \[a-c\] | trans | cis | cis |
| \[b-d\] | trans | cis | cis |
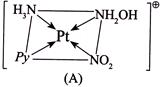
 For Y:- \[CN=6\] Geometry is octahedral. Three isomers are possible. I.
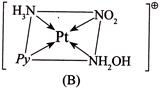
For Y:- \[CN=6\] Geometry is octahedral. Three isomers are possible. I. 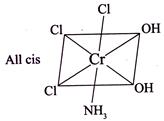 II.
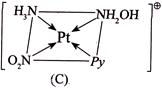
II. 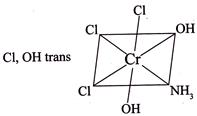 III.
III. 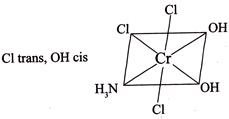
You need to login to perform this action.
You will be redirected in
3 sec
