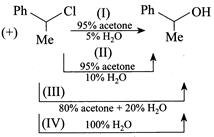
 Arrange the above reactions in the decreasing order of greater proportion of inverted product and select the correct answer.
Arrange the above reactions in the decreasing order of greater proportion of inverted product and select the correct answer.
A) I>II>III>IV
B) II>I>III>IV
C) III>II>I>IV
D) IV>III>II>I
Correct Answer: D
Solution :
[d] Inverted products means \[{{S}_{N}}2,\] which is faster in more polar protic solvent with neutral nucleophile \[({{H}_{2}}O)\]You need to login to perform this action.
You will be redirected in
3 sec
