A) \[C{{r}^{3+}}\]
B) \[M{{n}^{3+}}\]
C) \[F{{e}^{2+}}\]
D) \[C{{o}^{2+}}\]
Correct Answer: C
Solution :
[c] \[F{{e}^{2+}}:\] \[\mu =4.90\] B.M. corresponds to \[n=4\] which corresponds \[{{d}^{6}}\] configuration. In a weak field ligands, \[{{d}^{6}}\] configuration will have 4 unpaired electrons. In strong field ligands, \[{{d}^{6}}\]configuration will have no unpaired electrons with zero magnetic moment value. \[F{{e}^{2+}}\] have \[3{{d}^{6}}\] configuration. In weak field ligands: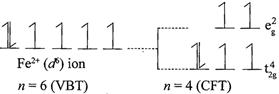 \[F{{e}^{2+}}:\]In strong field ligands:
\[F{{e}^{2+}}:\]In strong field ligands: 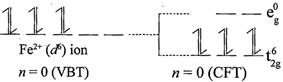
You need to login to perform this action.
You will be redirected in
3 sec
