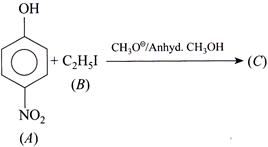
A) \[p-N{{O}_{2}}-{{C}_{6}}{{H}_{4}}-O{{C}_{2}}{{H}_{5}}\]
B) \[p-N{{O}_{2}}-{{C}_{6}}{{H}_{4}}-O-{{C}_{6}}{{H}_{4}}-N{{O}_{2}}-p\]
C) \[{{C}_{2}}{{H}_{5}}-O-C{{H}_{3}}\]
D) \[p-N{{O}_{2}}-{{C}_{6}}{{H}_{5}}-I\]
Correct Answer: C
Solution :
[c] \[C{{H}_{3}}{{O}^{\Theta }}\] acts as a base. It abstracts \[{{H}^{\oplus }}\] from p-nitrophenol to form\[p-N{{O}_{2}}-{{C}_{6}}{{H}_{4}}{{O}^{\Theta }}.\]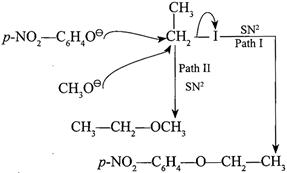 ' \[C{{H}_{3}}{{O}^{\Theta }}\] is a stronger nucleophile than \[p-N{{O}_{2}}-{{C}_{6}}{{H}_{4}}{{O}^{\Theta }},\]hence the product is obtained by path II. (Acidic character: \[p-N{{O}_{2}}-{{C}_{6}}{{H}_{4}}OH>C{{H}_{3}}OH\]) (Basic and nucleophilic character: \[p-N{{O}_{2}}-{{C}_{6}}{{H}_{4}}{{O}^{\Theta }}<C{{H}_{3}}{{O}^{\Theta }})\]
' \[C{{H}_{3}}{{O}^{\Theta }}\] is a stronger nucleophile than \[p-N{{O}_{2}}-{{C}_{6}}{{H}_{4}}{{O}^{\Theta }},\]hence the product is obtained by path II. (Acidic character: \[p-N{{O}_{2}}-{{C}_{6}}{{H}_{4}}OH>C{{H}_{3}}OH\]) (Basic and nucleophilic character: \[p-N{{O}_{2}}-{{C}_{6}}{{H}_{4}}{{O}^{\Theta }}<C{{H}_{3}}{{O}^{\Theta }})\]
You need to login to perform this action.
You will be redirected in
3 sec
