| I. p-Fluorophenol |
| II. p-Chlorophenol |
| III. p-Bromophenol |
| IV. p-Iodophenol |
A) \[II>I>III>IV\]
B) \[I>II>III>IV\]
C) \[IV>III>II>I\]
D) \[IV>III>I>II\]
Correct Answer: A
Solution :
[a]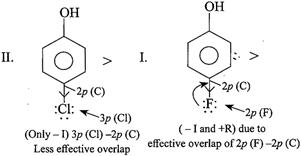
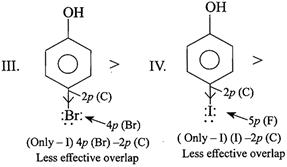 In case of F, both \[-I\] and \[+R\] effects are considered because of the effective overlap of \[2p(F)-2p(C)\]. But in other halogens, only \[-I\] effect is considered due to ineffective overlap. So net e-withdrawing power of\[Cl>F>Br>I\]. Hence, the acidic order is. \[II>I>III>IV\]
In case of F, both \[-I\] and \[+R\] effects are considered because of the effective overlap of \[2p(F)-2p(C)\]. But in other halogens, only \[-I\] effect is considered due to ineffective overlap. So net e-withdrawing power of\[Cl>F>Br>I\]. Hence, the acidic order is. \[II>I>III>IV\]
You need to login to perform this action.
You will be redirected in
3 sec
