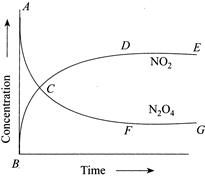
| (I) Reaction quotient has maximum value at point A. |
| (II) Reaction proceeds left to right at a point when \[[{{N}_{2}}{{O}_{2}}]=[N{{O}_{2}}]=[N{{O}_{2}}]=0.1M\] |
| (III) \[K=Q\]when point D or F is reached. |
A) I, II
B) II, III
C) II
D) I, II, III
Correct Answer: B
Solution :
[b] \[{{N}_{2}}{{O}_{4}}(g)2N{{O}_{2}}(g)\] \[{{K}_{c}}=4\] From graph, at point A, \[Q=0\] When \[[{{N}_{2}}{{O}_{4}}]=[N{{O}_{2}}]=0.1M\] \[\Rightarrow \,\,Q=\frac{{{[N{{O}_{2}}]}^{2}}}{[{{N}_{2}}{{O}_{4}}]}=0.1<{{K}_{c}}\Rightarrow \]Reaction is moving in forward direction. At point D or F, \[[N{{O}_{2}}]\]and \[[{{N}_{2}}{{O}_{4}}]\] is constant with time \[\Rightarrow \] Achievement of chemical equilibrium.You need to login to perform this action.
You will be redirected in
3 sec
