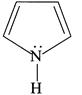
 (ii)
(ii) 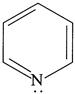 (iii)
(iii) 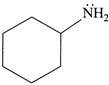 (iv)
(iv) 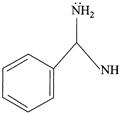
| Which of the following statements are correct? |
| (I) (i) and (ii) are aromatic and have equal basic strength. |
| (II) (i) is aromatic and (ii) is anti-aromatic, but (ii) is stronger base than (i). |
| (III) The order of basicity of the above compounds is\[(iv)>(iii)>(ii)>(i)\]. |
| (IV) The conjugate acid of (iv) is more stabilised than the conjugate acid of (ii). |
A) I, II
B) III, IV
C) I, III
D) II, IV
Correct Answer: B
Solution :
[b] (I) INCORRECT- CORRECT:- Both [a] and [b] are aromatic but (ii) is more basic than [a] (II) INCORRECT- CORRECT:- Both [a] and [b] are aromatic (III) In [d], lone pair of two N makes it more basic and does not delocalise in the benzene ring. In [c], no delocalisation of LP of \[\bar{e}'s\] on \[\overset{\bullet \,\bullet }{\mathop{N}}\,{{H}_{2}}\]. In [b], no delocalisation of LP of \[\bar{e}'s\] on N. In [a], delocalisation ofLP of \[\bar{e}'s\] on N via resonance. Hence, the order of basic character is:\[d>c>b>a\]. (IV) CORRECT statementYou need to login to perform this action.
You will be redirected in
3 sec
