A) 4.606 cal
B) \[\frac{0.2}{2.303}cal\]
C) 2 cal
D) None of these
Correct Answer: C
Solution :
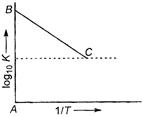
Rate constant, \[k=A{{e}^{-{{E}_{a}}/RT}}\] In \[k=\frac{-{{E}_{a}}}{RT}+In\,A\] \[2.303{{\log }_{10}}k=\frac{-{{E}_{a}}}{RT}+2.303{{\log }_{10}}A'\] \[{{\log }_{10}}k=\frac{-{{E}_{a}}}{2.303R}\cdot \frac{1}{T}+{{\log }_{10}}A'\] Now, \[\frac{-{{E}_{a}}}{2.303R}=\tan \theta \,=-\frac{1}{2.303}\] \[\therefore \] \[{{E}_{a}}=R=2\,cal\]You need to login to perform this action.
You will be redirected in
3 sec
