A) Linear shape
B) Angular shape
C) Trigonal planar shape
D) T-shape
Correct Answer: A
Solution :
Central \[\text{I}\]atom in \[{{\text{I}}_{3}}^{-}\]is \[s{{p}^{3}}d\]hybridised. Lone pairs occupy equatorial position.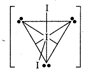
You need to login to perform this action.
You will be redirected in
3 sec
