A) 6 and 6
B) 2 and 6
C) 6 and 2
D) 8 and 8
Correct Answer: C
Solution :
\[\frac{Co-ordination\,no\,of\,cation}{Co-ordination\,no\,of\,anion}\,=\frac{ch\arg e\,of\,cation}{ch\arg e\,of\,anion}\] \[=\frac{3}{1}=3\] Now Co-ordination no. of A is as shown so of B is 2.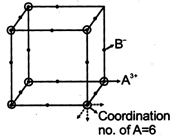
You need to login to perform this action.
You will be redirected in
3 sec
