| Column-I (Complex) | Column-II (Hybridisation) | ||
| (I) | \[{{[Au{{F}_{4}}]}^{-}}\] | (p) | \[ds{{p}^{2}}\] hybridisation |
| (II) | \[{{[Cu{{(CN)}_{4}}]}^{3-}}\] | (q) | \[s{{p}^{3}}\] hybridisation |
| (III) | \[[Co{{({{C}_{2}}{{O}_{4)3}}]}^{3-}}\] | (r) | \[s{{p}^{3}}{{d}^{2}}\] hybridisation |
| (IV) | \[{{[Fe{{({{H}_{2}}O)}_{5}}NO]}^{2+}}\] | (s) | \[{{d}^{2}}s{{p}^{3}}\] hybridisation |
A) I-q II-p III-r IV-s
B) I-p II-q III-s IV-r
C) I-p II-q III-r IV-s
D) I-q II-p III-s IV-s
Correct Answer: B
Solution :
(I) Au in +3 oxidation state with \[5{{d}^{8}}\] configuration has higher CFSE. So complex has \[ds{{p}^{2}}\] hybridisation and is diamagnetic. (II) Cu is in +1 oxidation state with 3d10 configuration and no (n - 1)d orbital is available for \[ds{{p}^{2}}\] hybridsation, so ns and np orbitals undergo \[s{{p}^{3}}\] hybridisation and complex is diamagnetic. (III) Co is in +3 oxidation state with 3d6 configuration has higher CFSE. So complex has diamagnetic and has \[{{d}^{2}}s{{p}^{3}}\] hybridisation. (IV) Fe is in +1 oxidation state and the complex is paramagnetic with three unpaired electrons.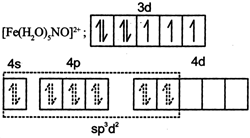
You need to login to perform this action.
You will be redirected in
3 sec
