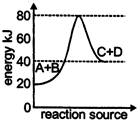
A) The activation energy of backward reaction is \[40\,kJ\]
B) \[\Delta \,H\] for forward reaction is \[20\,kJ\]
C) The forward reaction is endothermic
D) The activation energy for forward reaction is\[50\,kJ\]
Correct Answer: D
Solution :
Activation Energy for forward reaction =\[80-40=40\]kJYou need to login to perform this action.
You will be redirected in
3 sec
