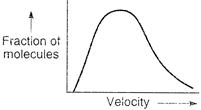
| (I) Oxygen gas at T temperature |
| (II) Hydrogen gas at 2T temperature |
| (III) Helium gas at 2T temperature |
| (IV) Sulphur dioxide gas at 2T temperature |
A) I and II
B) II and III
C) I and IV
D) III and IV
Correct Answer: C
Solution :
Graph having same M/T value will have same profile curve \[v=4\,m{{s}^{-1}}\] \[Mg\,\,\sin \,\,\theta \] \[Mg\,\,\sin \theta \times \frac{h}{2}Mg\,\cos \,\theta \,\times r\]\[\tan \theta =\frac{r}{h/2}\] \[\theta ={{45}^{o}},\] \[r=\frac{h}{2}\] Hence, they have the same profile curve.You need to login to perform this action.
You will be redirected in
3 sec
