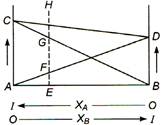
| 1. Plots AD and BC show that Raoult's law is obeyed for the solution in which B is a solvent and A is the solute and as well as for that in which A is solvent and B is solute. |
| 2. Plot CD shows that Dalton's law of partial pressure is observed by the binary solution of components A and B. |
| 3.\[EF+EG+EH\]; and AC and BD corresponds to the vapour pressure of the pure solvents A and B respectively. |
A) 1 and 2
B) 2 and 3
C) 1 and 3
D) 1, 2 and 3
Correct Answer: D
Solution :
By careful study of the graph it is quite obvious.You need to login to perform this action.
You will be redirected in
3 sec
