A) \[Na<Mg>Al<Si\]
B) \[Na>Mg>Al>Si\]
C) \[Na<Mg<Al>Si\]
D) \[Na>Mg>Al<Si\]
Correct Answer: A
Solution :
Ionisation energy increases from left to right in a period. However, exception occur between group - 2 and group -13 elements on account of stability of electronic configuration of valence shell.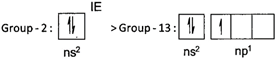 \[\Rightarrow \]The desired order is : \[Na<Mg>Al<Sl\]
\[\Rightarrow \]The desired order is : \[Na<Mg>Al<Sl\]
You need to login to perform this action.
You will be redirected in
3 sec
