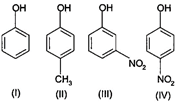
 The order of acidity is
The order of acidity is
A) \[III>IV>I>II\]
B) \[I>IV>III>II\]
C) \[II>I>III>IV\]
D) \[IV>III>I>II\]
Correct Answer: D
Solution :
Nitrogen group from para position exert electron withdrawing resonance effect, increases acidity of phenol the most. This is followed by meta nitrophenol in which nitro group exert electron withdrawing effect on acidity. \[C{{H}_{3}}-\] is an electron donating group, decreases acid strength. Hence, the overall order is \[IV>III>I>II\]You need to login to perform this action.
You will be redirected in
3 sec
