| For the two reactions \[\operatorname{I}:A\to B;II:C\to D\] following graph is obtained. |
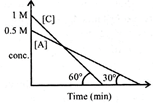 |
| Which of the following id true: |
A) If\[\left[ B \right]=\left[ A \right]\]then at the time \[\left[ D \right]=0.75M\]
B) If \[\left[ C \right]=\left[ A \right]\]then at that time \[\left[ B \right]>\left[ D \right]\]
C)
\[{\left( {{t}_{100percent}} \right)_{Reaction\,I}}={{\left( {{t}_{100percent}} \right)}_{Reaction\,II}}\]
D) \[\left[ A \right]=\left[ C \right]\]at \[\operatorname{t}=\frac{\sqrt{3}}{2}min\]
Correct Answer: A
Solution :
\[{{\left[ \operatorname{B} \right]}_{t}}={{\operatorname{K}}_{I}}\operatorname{t};0.25=\frac{1}{\sqrt{3}}\operatorname{t};t=0.25\sqrt{3}\] \[{{\left[ \operatorname{D} \right]}_{\operatorname{t}}}={{\operatorname{K}}_{II}}\operatorname{t}=\sqrt{3}\times 0.25\times \sqrt{3}=0.75\operatorname{M}\]You need to login to perform this action.
You will be redirected in
3 sec
