A) \[{{[CoC{{l}_{4}}]}^{2-}}-\text{tetrahedral}\]
B) \[[Co{{(PM{{e}_{3}})}_{4}}]-\text{tetrahedral}\]
C) \[{{[Cu{{(CN)}_{4}}]}^{3-}}-\text{tetrahedral}\]
D) \[{{[Fe{{(CO)}_{4}}]}^{2-}}-\text{square}\,\,\text{planar}\]
Correct Answer: D
Solution :
A, B and C are correctly, matched. [D] tetrahedral, CO stronger ligand, so pairing of electrons occurs.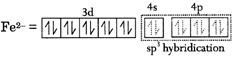
You need to login to perform this action.
You will be redirected in
3 sec
