A)
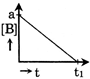
B)
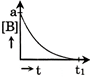
C)
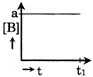
D)
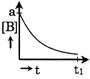
Correct Answer: B
Solution :
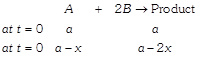 |
| \[{{[A]}_{t}}=a{{e}^{-kt}}\] |
| \[a-x=a{{e}^{-kt}}\] |
| \[\Rightarrow x=a\,\,(1-{{e}^{-\,kt}})\] |
| \[at\,\,t={{t}_{1}}\] \[\,a-x=\frac{a}{2}\] |
| \[x\]\[=\frac{a}{2}\] |
| \[[{{B}_{t}}]=a-2x\]\[=a-2a\,\,(1-{{e}^{-\,kt}})\]\[=a\,(2{{e}^{-\,kt}}-1)\] |
| \[at\,t=0\] \[[B]=a\] |
| \[at\,\,t={{t}_{1/2}}\] of \[a,[{{B}_{t}}]=a-2x\] |
| \[=a-a\] = 0 |
| Only graph [b] matches this. |
You need to login to perform this action.
You will be redirected in
3 sec
