A) resonance in be] but not in \[BF_{4}^{-}\]
B) \[p\pi \text{-}p\pi \] back bonding in \[BF_{4}^{-}\]but not in \[B{{F}_{3}}\]
C) \[p\pi \text{-}p\pi \] back bonding in \[B{{F}_{3}}\] but not in \[BF_{4}^{-}\]
D) \[p\pi \text{-}p\pi \] back bonding in \[B{{F}_{3}}\] but not in \[BF_{4}^{-}\]
Correct Answer: C
Solution :
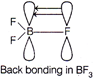
| In \[B{{F}_{3}}\]boron is \[s{{p}^{2}}\]hybridised. It has a vacant 2p-orbital, each fluorine atom in \[B{{F}_{3}}\] has completely filled unutilised 2p-orbitals. So, \[p\pi \text{-}p\pi \]back bonding occurs, where a lone pair of electrons of F-atoms gets transferred into vacant 2p-orbital. Therefore, B-F, bond has some double bond character. That is why all the 3B-F bonds are shorter than the usual single B-F bond. |
| Whereas, in \[[B{{F}_{4}}],\]boron is \[s{{p}^{3}}\text{-}\]hybridised and thus does not have empty 2p-orbital, so no such \[p\pi \text{-}p\pi \]back bonding can occur. \[BF_{4}^{-}\] has pure B-F bonds. Hence, B-F bond length in \[B{{F}_{3}}\] are shorter than \[BF_{4}^{-}.\] |
 |
You need to login to perform this action.
You will be redirected in
3 sec
