A) Isochoric
B) Lsobaric
C) Isothermal
D) Cyclic
Correct Answer: A
Solution :
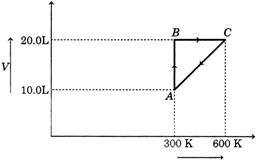
[a]| At A |
| Temp. = 300 K, volume = 10 L |
| Let pressure at this point \[={{p}_{1}}\]At C |
| Temp. = 600 K, volume = 20 L |
| Let the pressure at this point \[={{p}_{2}}\] |
| According to ideal gas, |
| pV = RT (For 1 mole) |
| \[R=\frac{pV}{T}\] |
| \[\therefore \]For system A, R \[=\frac{{{p}_{1}}\times 10}{300}\] |
| For system \[=\frac{{{p}_{2}}\times 20}{600}\] |
| \[=\frac{{{p}_{1}}\times 10}{300}=\frac{{{p}_{2}}\times 20}{600}\] |
| \[{{p}_{1}}={{p}_{2}}\] |
| Thus, it is an isochoric process, when system moves from A \[\to \] C. |
You need to login to perform this action.
You will be redirected in
3 sec
