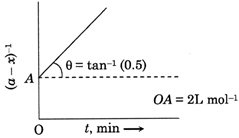
| Given is the graph between \[{{(a-x)}^{-1}}\] and time. |
 |
| Hence, rate at the start of the reaction is- |
A) \[1.25\,\,mol\,\,{{L}^{-1}}\,\,{{\min }^{-1}}\]
B) \[0.125\,\,mol\,\,{{L}^{-1}}\,\,{{\min }^{-1}}\]
C) \[0.5\,\,mol\,\,{{L}^{-1}}\,\,{{\min }^{-1}}\]
D) \[1.25\,\,mol\,\,{{\min }^{-1}}\]
Correct Answer: B
Solution :
[B]| since, the graph of t vs \[{{(a-x)}^{-1}}\]is a straight line, it must be a second order reaction. |
| \[\therefore K=\frac{1}{t}\left( \frac{1}{(a-x)}-\frac{1}{a} \right)\] |
| Or \[\frac{1}{a-x}=Kt+\frac{1}{a}\] |
| On comparing, slope |
| \[K=\tan \theta =0.5\,\,mo{{l}^{-1}}L\,{{\min }^{-1}}\] |
| \[OA=\frac{1}{a}=2\,\,L\,mo{{l}^{-1}}\] |
| Or \[a=0.5\,\,mol\,{{L}^{-1}}\] |
| Rate \[=K{{(a)}^{2}}=0.5\times {{(0.5)}^{2}}\] |
| \[=0.125\,mol\,{{L}^{-1}}{{\min }^{-1}}\] |
You need to login to perform this action.
You will be redirected in
3 sec
