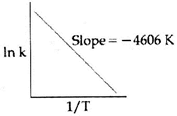
| For a certain reaction consider the plot of \[\ell nk\] versus 1/T given in the figure. If the rate constant of this reaction at 400 K is \[{{10}^{-5}}{{s}^{-1}},\]then the rate constant at 500 K is: |
 |
A) \[{{10}^{-6}}{{s}^{-1}}\]
B) \[2\times {{10}^{-4}}{{s}^{-1}}\]
C) \[{{10}^{-4}}{{s}^{-1}}\]
D) \[4\times {{10}^{-4}}{{s}^{-1}}\]
Correct Answer: C
Solution :
| \[\ell n=\ell nA-\frac{Ea}{RT}\]\[=\ell nA-\frac{4606}{T}\] |
| \[\ell n\left( \frac{k}{{{10}^{-5}}} \right)=\left( \frac{Ea}{R} \right)\times \frac{500-400}{500\times 400}\] |
| \[\ell n\left( \frac{k}{{{10}^{-5}}} \right)=4606\times \frac{1}{2000}=2.303=\ell n10\] |
| \[\ell n\left( \frac{k}{{{10}^{-5}}} \right)=\ell n10\] |
| \[k={{10}^{-4}}.\] |
You need to login to perform this action.
You will be redirected in
3 sec
