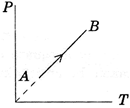
A) Work done on the gas is zero
B) Density of the gas is constant
C) Slope of line AB from the T-axis is directly proportional to the number of moles of the gas
D) All of the above
Correct Answer: D
Solution :
| P-T graph is a straight line passing through the origin. |
| Therefore V = constant |
| Work done on the gas, |
| Further, density of the gas, \[\rho =\frac{m}{V}\] |
| \[\therefore \rho \propto \frac{1}{V}\] |
| Since Volume of the gas is constant, density of the gas is constant. |
| \[PV=nRT\] |
| \[p=\left( \frac{nR}{V} \right)T\] |
| i.e., slope of P-T line is proportional to n. |
You need to login to perform this action.
You will be redirected in
3 sec
