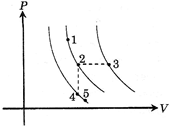
| A certain gas is taken to the five states represented by dots in the graph. The plotted lines are isotherms. Order of the most probable speed \[{{V}_{p}}\]of the molecules at these five states is - |
 |
A) \[{{V}_{P\text{ }at\text{ }3}}>{{V}_{P\text{ }at\text{ }1}}={{V}_{P\text{ }at\text{ }2}}>{{V}_{P\text{ }at\text{ }4}}={{V}_{P\text{ }at\text{ }5}}\]
B) \[{{V}_{P\text{ }at\text{ 1}}}>{{V}_{P\text{ }at\text{ 2}}}={{V}_{P\text{ }at\text{ 3}}}>{{V}_{P\text{ }at\text{ }4}}>{{V}_{P\text{ }at\text{ }5}}\]
C) \[{{V}_{P\text{ }at\text{ 3}}}>{{V}_{P\text{ }at\text{ 2}}}={{V}_{P\text{ }at\text{ 4}}}>{{V}_{P\text{ }at\text{ 1}}}>{{V}_{P\text{ }at\text{ }5}}\]
D) Insufficient information to predict the result.
Correct Answer: A
Solution :
Stages 1 and 2 are at same temperature also stages 4 and 5 are at same temperature. As, \[{{V}_{P}}\] is more at higher temperature and same at all stages at equal temperature. \[\therefore {{V}_{P3}}>{{V}_{P1}}={{V}_{P2}}>{{V}_{P4}}={{V}_{P5}}\] Hence [A].You need to login to perform this action.
You will be redirected in
3 sec
