A) four \[2C-2{{e}^{-}}\]bonds and four \[3C-2{{e}^{-}}\]bonds
B) two \[2C-2{{e}^{-}}\]bonds and two \[3C-2{{e}^{-}}\]bonds
C) two \[2C-2{{e}^{-}}\]bonds and four \[3C-2{{e}^{-}}\]bonds
D) four \[2C-2{{e}^{-}}\]bonds and two\[3C-2{{e}^{-}}\]bonds
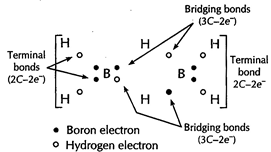
Correct Answer: D
Solution :
| The structure of diborane \[\left( {{B}_{2}}{{H}_{6}} \right)\] has four \[2C-2{{e}^{-}}\] bonds and two \[3C-2{{e}^{-}}\]bonds. |
 |
You need to login to perform this action.
You will be redirected in
3 sec
