A) \[NaCl+KN{{O}_{3}}\xrightarrow{{}}NaN{{O}_{2}}+KCl\]
B) \[Ca{{C}_{2}}{{O}_{4}}+2HCl\xrightarrow{{}}CaC{{l}_{2}}+{{H}_{2}}{{C}_{2}}{{O}_{4}}\]
C) \[Ca\,{{(OH)}_{2}}+2N{{H}_{4}}Cl\xrightarrow{{}}CaC{{l}_{2}}+2N{{H}_{3}}+2{{H}_{2}}O\]
D) \[2K\,[Ag\,{{(CN)}_{2}}+Zn\xrightarrow{{}}2Ag+{{K}_{2}}[Zn\,{{(CN)}_{4}}]\]
Correct Answer: D
Solution :
[a] 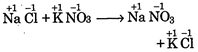 |
[b] 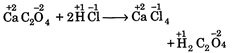 |
[c] 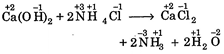 |
| In all these cases during reaction, there is no change in oxidation state of ion or molecule or constituent atom, thus these are simply ionic reactions. |
| [d] |
| \[\begin{align} & 2K[Ag{{\left( CN \right)}_{2}}]+Zn\to 2Ag \\ & +{{K}^{2}}[Zn{{\left( CN \right)}_{4}}] \\ \end{align}\] |
| \[A{{g}^{+}}\xrightarrow{{}}Ag\]gain of \[{{e}^{-}},\]reduction |
| \[Zn\xrightarrow{{}}Z{{n}^{2+}}\] loss of \[{{e}^{-}},\] oxidation. |
| As both oxidation and reduction takes place simultaneously. |
| Thus, it is a redox reaction. |
You need to login to perform this action.
You will be redirected in
3 sec
