| A \[0.150\]mole sample of an ideal gas is allowed expand at 294 K from 10.00 atm to 1.00 atm. |
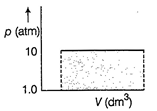 |
| If external pressure is kept constant at 1.00 atm work done is |
A) \[-300J\]
B) \[-3.26d{{m}^{3}}atm\]
C) Both [a] and [b]
D) None of these
Correct Answer: C
Solution :
| \[{{V}_{\left( initial \right)}}=\frac{nRT}{p}\]\[=\frac{0.150\times 0.0821\times 294}{10}=0.362d{{m}^{3}}\] |
| \[{{V}_{\left( final \right)}}=\frac{0.150\times 0.0821\times 294}{10}=0.362d{{m}^{3}}\] |
| \[\therefore \,W=-p\Delta V=-1\times \left( 3.62-0.362 \right)\] |
| \[=-3.26d{{m}^{3}}\,atm\]Thus, [b] is correct. |
| \[d{{m}^{3}}\,atm\]can be converted into joules as follows\[=-\frac{-3.26\times 8.314}{0.0821}J=-330J\]Thus [c] is correct. |
You need to login to perform this action.
You will be redirected in
3 sec
