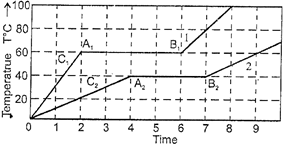
| Two solid bodies of equal mass m initially at \[T=0{}^\circ C\] are heated at a uniform and same rate under identical conditions. The temperature of the first object with latent heat \[{{L}_{1}}\] and specific heat capacity in solid state \[{{C}_{1}}\] changes according to graph 1 on the diagram. The temperature of the second object with latent heat \[{{L}_{2}}\] and specific heat capacity in solid state \[{{C}_{2}}\] changes according to graph 2 on the diagram. |
| Based on what is shown on the graph, the latent heats \[{{L}_{1}}\] and \[{{L}_{2}},\] and the specific heat capacities \[{{C}_{1}}\] and \[{{C}_{2}}\] in solid state obey which of the following relationships: |
 |
A) \[{{L}_{1}}>{{L}_{2}}\,\,;\,\,{{C}_{1}}<{{C}_{2}}\]
B) \[{{L}_{1}}<{{L}_{2}}\,\,;\,\,{{C}_{1}}<{{C}_{2}}\]
C) \[{{L}_{1}}>{{L}_{2}}\,\,;\,\,{{C}_{1}}>{{C}_{2}}\]
D) \[{{L}_{1}}<{{L}_{2}}\,\,;\,\,{{C}_{1}}>{{C}_{2}}\]
Correct Answer: A
Solution :
| If heat is supplied at constant rate P, then \[Q=P\Delta \,t\] and as during change of state Q = mL, so, \[mL=P\Delta \,t\] |
| i.e., \[L=\left[ \frac{P}{m} \right]\Delta \,t=\frac{P}{m}\](length of line AB) Hence \[{{L}_{1}}>{{L}_{2}}\] |
| i.e., the ratio of latent heat of fusion of the two substances are in the ratio 3 : 4. In the portion OA the substance is in solid state and its temperature is changing. \[\Delta \,Q=mC\,\Delta T\] and \[\Delta \,Q=P\Delta \,t\] So, \[\frac{\Delta \,T}{\Delta \,t}=\frac{P}{mC}\] or |
| slope\[=\frac{P}{mS}=\left[ \text{as}\frac{\Delta \,T}{\Delta \,t}=\text{slope} \right]\] |
You need to login to perform this action.
You will be redirected in
3 sec
