A) \[\alpha =\sqrt{{{K}_{eq}}/c(x+y)}\]
B) \[\alpha =\sqrt{{{K}_{eq}}c/(xy)}\]
C) \[\alpha ={{\left( {{K}_{eq}}/{{c}^{x+y-1}}{{x}^{2}}{{y}^{2}} \right)}^{1/\left( x+y \right)}}\]
D) \[\alpha =\left( {{K}_{eq}}/cxy \right)\]
Correct Answer: C
Solution :
| the week electrolyte AxBy dissociates as follows |
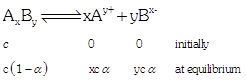 |
| Where, a =degree of dissociation |
| c = concentration |
| \[{{K}_{eq}}=\frac{{{[{{A}^{y+}}]}^{x}}[{{B}^{x-}}]y}{[{{A}_{x}}{{B}_{y}}]}\] |
| \[=\frac{{{[xc\alpha ]}^{x}}{{[yc\alpha ]}^{y}}}{c(1-\alpha )}\] |
| \[=\frac{{{x}^{x}}.{{c}^{x}}.{{a}^{x}}.{{y}^{y}}.{{c}^{y}}.{{a}^{y}}}{c}\] \[[\because 1-\alpha \approx 1]\] |
| \[={{x}^{x}}.{{y}^{y}}.{{\alpha }^{x+y}}.{{c}^{x+y-1}}\] |
| \[{{\alpha }^{x+y}}=\frac{{{K}_{eq}}}{{{x}^{x}}.{{y}^{y}}.{{c}^{x+y-1}}}\] |
| \[\alpha ={{\left( \frac{{{K}_{eq}}}{{{x}^{x}}.{{y}^{y}}.{{c}^{x+y-1}}} \right)}^{\left( \frac{1}{x+y} \right)}}\] |
You need to login to perform this action.
You will be redirected in
3 sec
