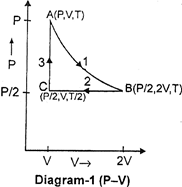
| Three moles of an ideal gas \[[{{C}_{p}}=7/2\,\,R]\] at pressure 'P' and temperature 'T' is isothermally expanded to twice its initial volume. It is then compressed at constant pressure to its original volume. Finally the gas is brought at constant volume to its original pressure P. Consider the following diagrams. \[P-V\]and \[P-T\] diagrams for the processes |
 |
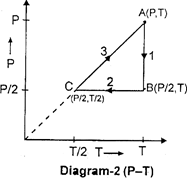 |
A) Only diagram-1 is correct
B) Only diagram-2 is correct
C) Both diagrams are correct
D) No diagram is correct
Correct Answer: C
Solution :
Not availableYou need to login to perform this action.
You will be redirected in
3 sec
