| One mole of an ideal monatomic gas has initial temperature \[{{\operatorname{T}}_{0}}\], is made to go through the cycle \[abca\] shown in the given figure. If U denotes the internal energy, then choose the correct alternative. |
 |
A) \[{{U}_{c}}>{{U}_{b}}>{{U}_{a}}\]
B) \[{{U}_{c}}-{{U}_{b}}=5R{{T}_{0}}\]
C) \[{{U}_{c}}-{{U}_{a}}=\frac{9R{{T}_{0}}}{4}\]
D) \[{{U}_{b}}-{{U}_{a}}=\frac{3R{{T}_{0}}}{4}\]
Correct Answer: A
Solution :
| One mole of an ideal monatomic gas (initial temperature\[{{T}_{0}}\]) is made to go through the cycle \[abca\]shown in Fig. U denotes the internal energy. |
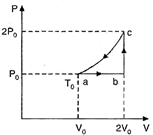 |
| For the process \[ab,\frac{{{P}_{0}}{{V}_{0}}}{{{T}_{0}}}=\frac{2{{P}_{0}}{{V}_{0}}}{{{T}_{0}}}\] |
| \[{{\operatorname{T}}_{b}}=2{{T}_{0}}\] |
| \[{{T}_{b}}>{{T}_{a}}\Rightarrow {{U}_{b}}>{{U}_{a}}\] |
| \[{{\operatorname{U}}_{b}}-{{U}_{a}}={{C}_{v}}\Delta T =\frac{3R}{2}\left( 2{{T}_{0}}-{{T}_{0}} \right)=\frac{3R{{T}_{0}}}{2}\] |
| For the process bc, |
| \[\frac{{{P}_{0}}\left( 2{{V}_{0}} \right)}{2{{T}_{0}}}=\frac{2{{P}_{0}}\left( 2{{V}_{0}} \right)}{{{T}_{C}}}\Rightarrow {{\operatorname{T}}_{C}}=4{{T}_{0}}\] |
| \[{{T}_{C}}>{{T}_{0}}\] |
| \[{{U}_{c}}-{{U}_{b}}=\frac{3R}{2}\left( 4{{T}_{0}}-2{{T}_{0}} \right)=3R{{T}_{0}}\] |
| For the process ca, |
| \[{{U}_{c}}-{{U}_{a}}=\frac{3R}{2}\left( 4{{T}_{0}}-{{T}_{0}} \right)=\frac{9R{{T}_{0}}}{2}\] |
You need to login to perform this action.
You will be redirected in
3 sec
