| In the following reactions, |
(i) 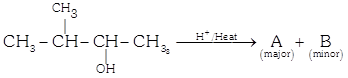 |
| (ii) \[\operatorname{A}\xrightarrow[in\,absence\,of\,peroxide]{HBr,dark}\underset{\left( major \right)}{\mathop{\operatorname{C}}}\,+\underset{\left( minoor \right)}{\mathop{\operatorname{D}}}\,\] |
| The major product [A] and [C] are respectively: |
A)
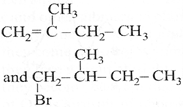
B)
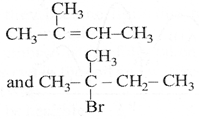
C)
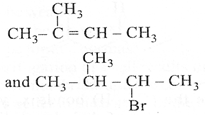
D)
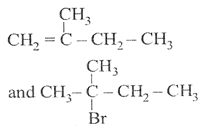
Correct Answer: B
Solution :
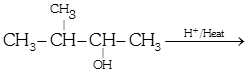 |
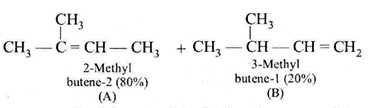 |
| In this case dehydration is governed by Saytzeff's rule, according to which hydrogen is preferentially eliminated from the carbon atom with fewer number of hydrogen atoms i.e., poor becomes poorer. |
| Thus, 2-methyl butene-2 is the major product. |
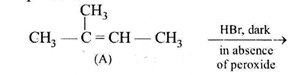  |
| This reaction is governed by Markovnikov's rule, according to which when an unsymmetrical reagent e.g. HBr adds to an unsymmetrical alkene, then the negative part of the reagent is added to that carbon atom of the double bond which bears the least number of hydrogen atom. Thus, in above case 2-methyl 2-bromobutane will be the major product. |
You need to login to perform this action.
You will be redirected in
3 sec
