A) 0.85 K
B) -3.53K
C) 0 K
D) -0.38K
Correct Answer: B
Solution :
| Given mass of solute = 8.1 g |
| Mass of solvent = 100 g |
| For \[HBr\] |
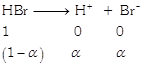 |
| \[\alpha =90%\text{ }=0.9\] |
| \[i=-1+\alpha =1+0.9=1.9\] |
| \[\Delta {{T}_{f}}={{K}_{f}}\times m\times i\] |
| \[=1.86\times \frac{moles\,of\,solute}{mass\,of\,solvent\,in\,kg}\times 1.9\] |
| \[=1.86\times \frac{8.1/81}{100/1000}\times 1.9\] |
| \[=1.86\times 1\times 1.9=3.534K\] |
| \[{{\operatorname{T}}_{f}}=T_{f}^{{}^\circ }-\Delta {{\operatorname{T}}_{f}}\] |
| or \[{{\operatorname{T}}_{f}}=0-3.534K\] |
| \[\therefore \,\,\,{{\operatorname{T}}_{f}}=-3.534K\] |
You need to login to perform this action.
You will be redirected in
3 sec
