| Consider the following reaction: \[M{{X}_{4}}+X{{'}_{2}}\xrightarrow{{}}M{{X}_{4}}X{{'}_{2}}\] |
| If atomic number of M is 52 and X and X' are halogens and X' is more electronegative than X. Then choose correct statement regarding given information: |
A) Both X' atoms occupy axial positions which are formed by overlapping of p and \[d\]-orbitals only
B) All M - X bond lengths are identical in both \[{{\operatorname{MX}}_{4}}\] and \[{{\operatorname{MX}}_{4}}X'{{ }_{2}}\] compounds
C) Central atom 'M' does not use non-axial set of \[d\]-orbital in hybridization of final product.
D) Hybridization of central atom 'M' remains same in both reactant and final product.
Correct Answer: C
Solution :
| \[{{\operatorname{MX}}_{4}}+{{X}_{2}}\xrightarrow{{}}M{{X}_{4}}X_{2}^{'}(M=Te)\] |
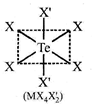 |
| Hybridization of \[\operatorname{Te}:s{{p}^{3}}d\] |
| In the above compound orbitals used hybridization of Te are oxial t.e\[s,{{p}_{x}}^{,}{{p}_{y}},{{p}_{z}}^{,}\] |
| \[{{d}_{{{x}^{2}}-{{y}^{2}}}},{{d}_{{{z}^{2}}}}\] |
| All M?X bond lengths are identical. |
| Now, |
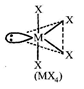 |
| Hybridization of Te: \[s{{p}^{3}}{{d}^{2}}\] |
| In the above comopound orbitals used in the hybridization of Te are \[s,{{p}_{x}},{{p}_{y}},{{p}_{z}},{{d}_{{{z}^{2}}}}.\] |
| All M?X bond lengths are not identical, |
You need to login to perform this action.
You will be redirected in
3 sec
