A) \[{{H}_{2}}O>{{H}_{2}}{{O}_{2}}>HF>{{H}_{2}}S\]
B) \[HF>{{H}_{2}}{{O}_{2}}>{{H}_{2}}O>{{H}_{2}}S\]
C) \[HF>{{H}_{2}}O>{{H}_{2}}S>{{H}_{2}}{{O}_{2}}\]
D) \[HF>{{H}_{2}}O>{{H}_{2}}{{O}_{2}}>{{H}_{2}}S\]
Correct Answer: D
Solution :
| Strength of H-bonding is higher in \[{{H}_{2}}O,\]than \[{{H}_{2}}{{O}_{2}}\] because the amount of formal negative charge on oxygen atom in case of water is more than that of \[{{H}_{2}}{{O}_{2}}.\] |
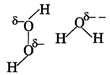 |
You need to login to perform this action.
You will be redirected in
3 sec
