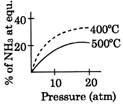
A)
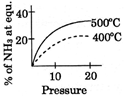
B)
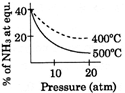
C)
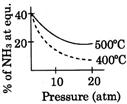
D)
Correct Answer: A
Solution :
[A] \[{{N}_{2}}(g)+3\,{{H}_{2}}(g)\,\,\,\,2N{{H}_{3}}(g);\Delta \,H<0\] By Le- Chatelier principle, higher pressure and lower temperature favour the forward reaction, i.e. higher % of \[N{{H}_{3}}.\]You need to login to perform this action.
You will be redirected in
3 sec
