A) both square planar
B) tetrahedral and square planar respectively
C) both tetrahedral
D) square planar and tetrahedral respectively
Correct Answer: C
Solution :
[c]| \[Ni\,{{(CO)}_{4}};\]Oxidation state of \[Ni=0\] |
| \[Ni=I{{s}^{2}},2{{s}^{2}}2{{p}^{6}},3{{s}^{2}}3{{p}^{6}},4{{s}^{2}},3{{d}^{8}}\] |
| As \[CO\]is a strong ligand and hence pairing of electrons occurs. |
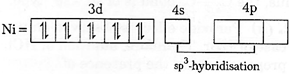 |
| Oxidation state of \[Ni\] in \[[Ni\,{{(PP{{h}_{3}})}_{2}}]\,C{{l}_{2}}\] is \[x+0\times 2+(-2)=0\] |
| \[x=+\,2\] |
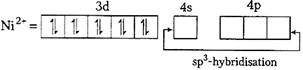 |
| As both the complexes has \[s{{p}^{3}}\text{-}\]hybridisation, thus their geometry is tetrahedral. |
You need to login to perform this action.
You will be redirected in
3 sec
