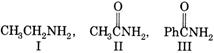 Basic nature of above compounds is in the order as:
Basic nature of above compounds is in the order as:
A) I > II > III
B) I > II > III
C) II > III > I
D) III > II > I
Correct Answer: A
Solution :
[a]| Basic nature of a compound depends upon the ease with which it can donate its unshared pair of electrons. |
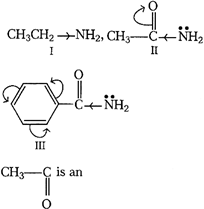 |
| I. electron donating group. (Maximum basic) |
| II. electron withdrawing. The lone pair of N is involved in resonance with \[C{{H}_{3}}CO.\] |
| III. Lone-pair of N-atom is also used in delocalization of \[\pi -\]electrons, resonance of carbonyl benzene nucleus (least basic) |
| Thus, the correct order of basic nature of given compounds are as follows: I > II > III. |
You need to login to perform this action.
You will be redirected in
3 sec
